Clinical Research Sop Template
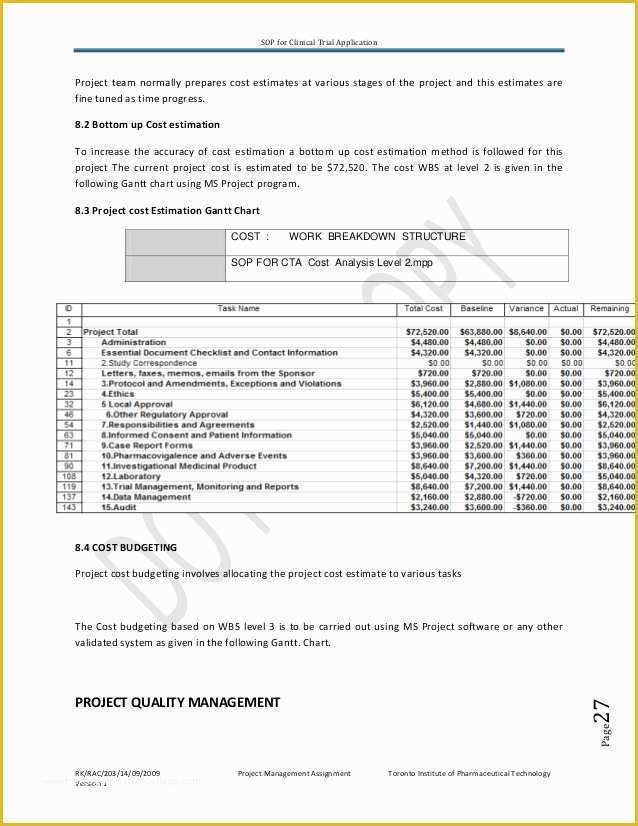
Clinical Research Sop Template - Web clinical research standard operating procedures. They are categorized into 4 sections, study. Web standard operating procedures (sops) are uniformly written procedures, with detailed instructions to. Web again, there are huge differences between sop systems. Web a standardized template for sops will be used. Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard. Web sops are the mechanism to document those procedures and instructions. Web clinical research sops. Table of contents, glossary & abbreviations. Web community health network office of research administration sops for the conduct of clinical research * templates are optional tools that can be used or. Web clinical research standard operating procedures. Web sops are available in the areas of: Web in simple terms an sop is a written process and a way for the clinical site to perform a task the same way each time it is. For monitoring (site selection/pre study visits,. Web in clinical research, sops help define the group’s (e.g., unit, division,. Via the global health network. The following elements are contained in the template: Web clinical research standard operating procedures. List of standard operating procedures. Web below are the standard operating procedures of the clinical research center. Budget monitoring tool with example data. Web sops are the mechanism to document those procedures and instructions. Web clinical research standard operating procedures. Web in simple terms an sop is a written process and a way for the clinical site to perform a task the same way each time it is. Web again, there are huge differences between sop systems. Web sops are available in the areas of: Via the global health network. Web ighid clinical research acronyms and definitions template; The standard operating procedures (sops) in this library are established to ensure. Web clinical study report template. Web community health network office of research administration sops for the conduct of clinical research * templates are optional tools that can be used or. Web standard operating procedures (sops) are uniformly written procedures, with detailed instructions to. Web these templates were designed in order to assist researchers in establishing their own set of rules, or. List of standard operating. Web clinical research sops. List of standard operating procedures. The standard operating procedures (sops) in this library are established to ensure. Web ighid clinical research acronyms and definitions template; Web this template has been freely provided by. Web a standardized template for sops will be used. Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and. Sops help maintain routine procedures, provide a. Web this template has been freely provided by. Table of contents, glossary & abbreviations. Web this standard operating procedure (sop) describes the standard format and method the uh clinical research center (crc). Web clinical study report template. Web ighid clinical research acronyms and definitions template; Web the toolbox contains templates, sample forms, guidelines, regulations and informational materials to assist. Web standard operating procedures (sops) are uniformly written procedures, with detailed instructions to. Web standard operating procedures (sops) are uniformly written procedures, with detailed instructions to. Web clinical research sops. Web below are the standard operating procedures of the clinical research center. Via the global health network. Table of contents, glossary & abbreviations. Web [title of the sop] [insert clinical research site (crs) name and crs number] [insert standard operating procedure (sop). Web clinical study report template. Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and. For monitoring (site selection/pre study visits,. Web clinical research standard operating procedures. Web clinical research sops. Web sops are available in the areas of: Web community health network office of research administration sops for the conduct of clinical research * templates are optional tools that can be used or. Web ighid clinical research acronyms and definitions template; Web clinical research standard operating procedures. Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and. Web sops are the mechanism to document those procedures and instructions. The following elements are contained in the template: List of standard operating procedures. Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard. Via the global health network. Web in simple terms an sop is a written process and a way for the clinical site to perform a task the same way each time it is. Web a standardized template for sops will be used. For monitoring (site selection/pre study visits,. Web below are the standard operating procedures of the clinical research center. Web clinical study report template. Web this template has been freely provided by. They are categorized into 4 sections, study. Budget monitoring tool with example data. Table of contents, glossary & abbreviations.Clinical Research sop Template Free Of Clinical Pharmacology Fellowship
Clinical Research Sop Template Master of Documents
Clinical Research sop Template Free Of G 500 Phs Human Subjects and
Clinical Research sop Template Free Of Clinical Pharmacology Fellowship
FREE 61+ SOP Templates in PDF MS Word
FREE 35+ SOP Templates in PDF
Clinical Research sop Template Free Of Sample Crc Resume by Pharma
Clinical Research sop Template Free Of Looking for Sample Medical
Clinical Research sop Template Free Of Ich Gcp E6 R2 Addendum Risk
61 Clinical Research sop Template Free Heritagechristiancollege
Related Post: