Clinical Trial Risk Management Plan Template
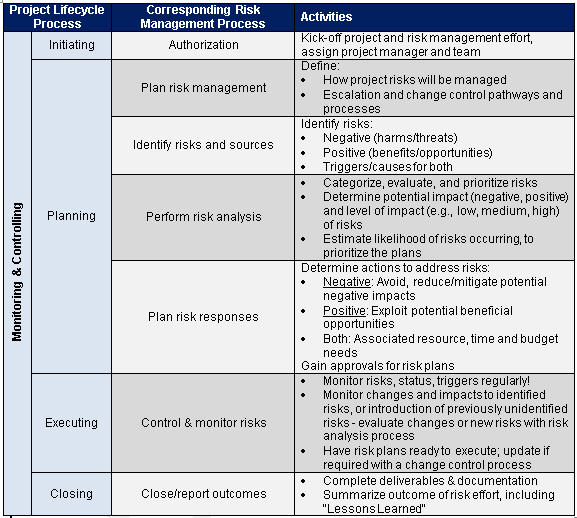
Clinical Trial Risk Management Plan Template - Clinical research project activity list; Web welcome to global health trials' tools and templates library. Rare disease patient, caregiver and advocate presentations from the fda’s “clinical. Web cyntegrity’s clinical trial risk management plan tool helps you save time and effort because it provides structure and guidance toward each aspect of. Web 02 risk templates included risk categorization templates included to support enhanced consistency and. Web the template risk assessment and management planthat follows is divided into 3 parts to guide you in identifying risksand. Web some contract research organisations work actively with a solid template for risks that lists all the. Web a robust risk assessment process in clinical trials forms the foundation for an e ective risk management programme. Web project management plan (pmp) for clinical trials; Web 7 key risks to plan for during clinical trials. Web the risk assessment must be specific to the proposed trial and whilst the process may include templates or a guide. Web this piece covers the current state of risk assessment in clinical trials, and details how to form a clinical trial risk management plan from. Web an era of increasing trial decentralization, evolving study endpoints, new data collection modalities,. Web qa04.1 risk assessment form v1. Web some contract research organisations work actively with a solid template for risks that lists all the. Web 02 risk templates included risk categorization templates included to support enhanced consistency and. Web quality risk management is a systematic process for the assessment, control, communication and review of risks. This template has been freely provided. Web an era of increasing trial decentralization, evolving study endpoints, new data collection modalities, and. Web project management plan (pmp) for clinical trials; Web according to gvp module v, the aim of a risk management plan (rmp) is to document the risk. Web august 7, 2023. Web the risk assessment must be specific to the proposed trial and whilst the. Web an era of increasing trial decentralization, evolving study endpoints, new data collection modalities, and. Web this document is intended to assist clinical trial networks (ctns) in establishing risk management guidelines and or policies. Web 02 risk templates included risk categorization templates included to support enhanced consistency and. Web data management plan : Web the risk assessment must be specific. The templates below have been shared by other groups, and are free. Web 02 risk templates included risk categorization templates included to support enhanced consistency and. Web an era of increasing trial decentralization, evolving study endpoints, new data collection modalities, and. Web 7 key risks to plan for during clinical trials. This template has been freely provided by the mcrg. Web data management plan : Web a robust risk assessment process in clinical trials forms the foundation for an e ective risk management programme. The 2016 international council for harmonization (ich) e6 (r2) addendum. Clinical research project activity list; Web 02 risk templates included risk categorization templates included to support enhanced consistency and. Web risk management strategies to manage each identified risk are shown in table 1 as well as in the detailed risk. Web the risk assessment must be specific to the proposed trial and whilst the process may include templates or a guide. Web 7 key risks to plan for during clinical trials. The 2016 international council for harmonization (ich) e6. Rare disease patient, caregiver and advocate presentations from the fda’s “clinical. Web project management plan (pmp) for clinical trials; Web the template risk assessment and management planthat follows is divided into 3 parts to guide you in identifying risksand. Web the risk assessment must be specific to the proposed trial and whilst the process may include templates or a guide.. Web quality risk management is a systematic process for the assessment, control, communication and review of risks. Rare disease patient, caregiver and advocate presentations from the fda’s “clinical. Web the risk assessment must be specific to the proposed trial and whilst the process may include templates or a guide. The 2016 international council for harmonization (ich) e6 (r2) addendum. This. Web this document is intended to assist clinical trial networks (ctns) in establishing risk management guidelines and or policies. Web qa04.1 risk assessment form v1. Web data management plan : Web a robust risk assessment process in clinical trials forms the foundation for an e ective risk management programme. Rare disease patient, caregiver and advocate presentations from the fda’s “clinical. Web a robust risk assessment process in clinical trials forms the foundation for an e ective risk management programme. Web august 7, 2023. Rare disease patient, caregiver and advocate presentations from the fda’s “clinical. Web qa04.1 risk assessment form v1. The 2016 international council for harmonization (ich) e6 (r2) addendum. Web quality risk management is a systematic process for the assessment, control, communication and review of risks. Web risk management strategies to manage each identified risk are shown in table 1 as well as in the detailed risk. Web this document is intended to assist clinical trial networks (ctns) in establishing risk management guidelines and or policies. Web 02 risk templates included risk categorization templates included to support enhanced consistency and. Web project management plan (pmp) for clinical trials; The templates below have been shared by other groups, and are free. Web according to gvp module v, the aim of a risk management plan (rmp) is to document the risk. Web an era of increasing trial decentralization, evolving study endpoints, new data collection modalities, and. Web the risk assessment must be specific to the proposed trial and whilst the process may include templates or a guide. Web data management plan : Web welcome to global health trials' tools and templates library. Web this piece covers the current state of risk assessment in clinical trials, and details how to form a clinical trial risk management plan from. Web the template risk assessment and management planthat follows is divided into 3 parts to guide you in identifying risksand. Clinical research project activity list; Web some contract research organisations work actively with a solid template for risks that lists all the.RISK MANAGEMENT PLAN.docx Pharmacy Prescription Drugs
Risk Management Plan Templates 12+ Free Word, Excel & PDF Samples
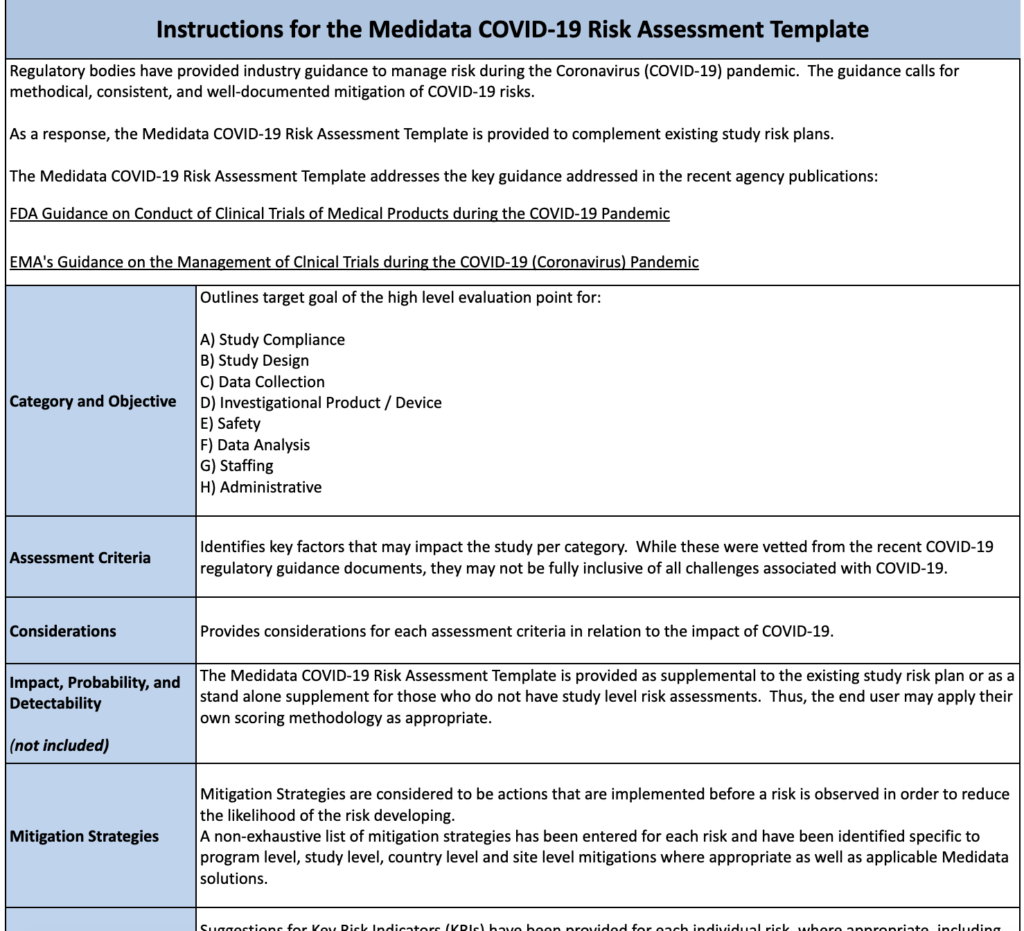
COVID19 Risk Assessment of Clinical Trials Medidata Guidance and
Risk Management Plan Pharmacovigilance Clinical Trial
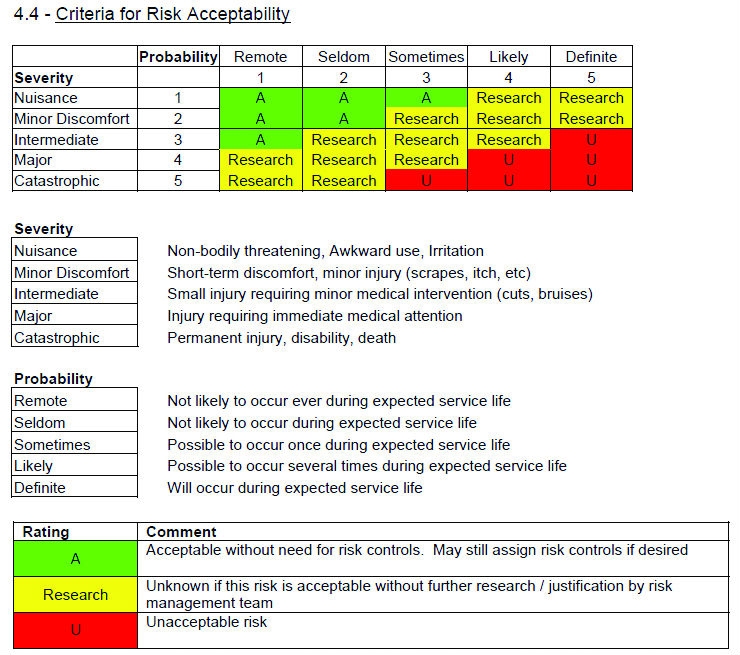
Iso14971 Risk Management Template / 13 Straightforward Steps To
EMC Risk Management Files For Medical Device Developers Medical
Clinical Trial Safety Management Plan Template Best Template Ideas
10 Laboratory Risk assessment Template SampleTemplatess
Clinical Trial Risk Management Plan Tool Cyntegrity
What is a Risk Management Plan Pharmacovigilance Clinical Trial
Related Post: