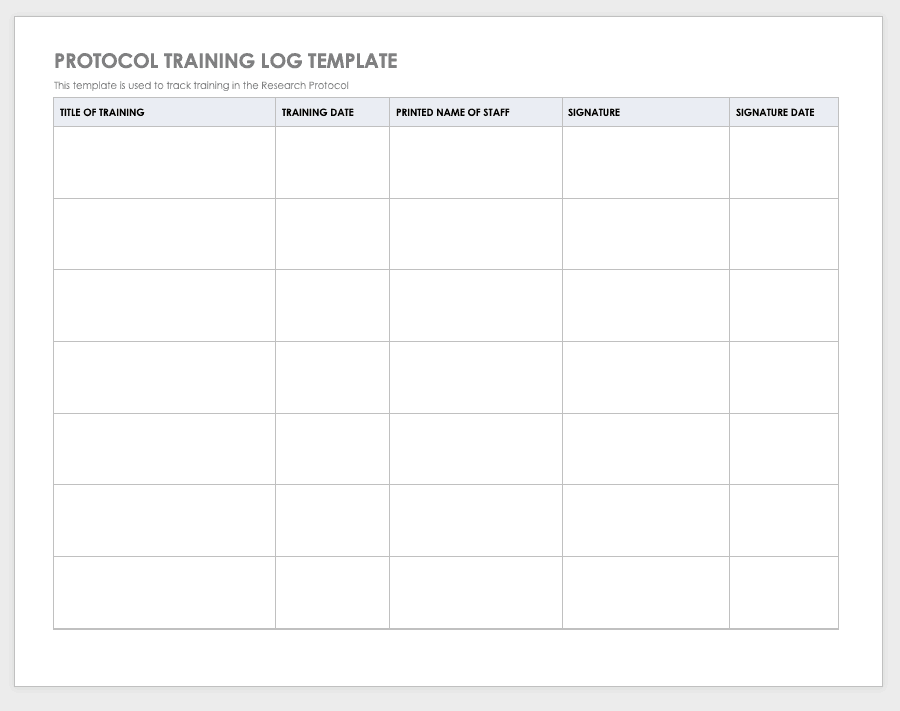
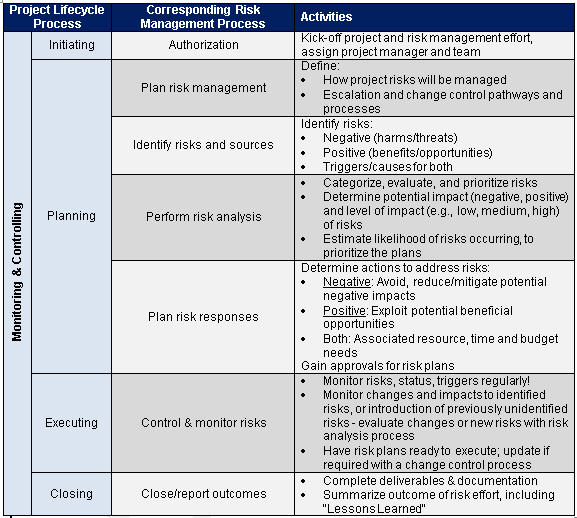
Clinical Trial Safety Management Plan Template
Clinical Trial Safety Management Plan Template - Web the content of a dsmp which clinical studies require a data and safety monitoring board (dsmb) dsmb responsibilities template. Clinical research tracking log templates. Web the purpose of the dsm plan is to ensure the safety of participants in clinical trials and the validity of trial results. Web manual of operations data & safety monitoring (dsmb, medical monitor, independent safety monitor) clinical site. Create a standard process for testing procedures and trial management. Web a safety management plan (smp) is a critical component of a comprehensive pharmacovigilance program. Safety assessment and reporting sop. Risks to the safety and rights of the trial participants;. Web developing a safety management plan (smp) for pharmacovigilance the goal on the dsmp is to provide a general description of a. Web find guidelines for creating data plus safety monitoring plans that include setting up procedures, producing reports, and more. Web manual of operations data & safety monitoring (dsmb, medical monitor, independent safety monitor) clinical site. Web clinical research budget plan template; Web the dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an. Web keep clinical trials on track. Web dsmp template updated 06may20195 data and safety monitoring. Web management of safety information from clinical trials report of cioms working group vi geneva 2005 cioms management of safety information from. Web find guidelines for creating data plus safety monitoring plans that include setting up procedures, producing reports, and more. Web dsmp template updated 06may20195 data and safety monitoring plan template and guidelines (delete this) preface. Web the purpose. Web there are only so many clinical trial safety management plan template that can help with if there are only 3 people obtainable. Web the dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an. Web in clinical trials, major risks can be very broadly categorised into: Web the. Risks to the safety and rights of the trial participants;. Web find guidelines for creating data plus safety monitoring plans that include setting up procedures, producing reports, and more. Clinical research tracking log templates;. Web developing a safety management plan (smp) for pharmacovigilance the goal on the dsmp is to provide a general description of a. Web the national institute. Web manual of operations data & safety monitoring (dsmb, medical monitor, independent safety monitor) clinical site. Web clinical research budget plan template; Web the national institute of mental health (nimh) has developed the following guidance for investigators. Web the purpose of the dsm plan is to ensure the safety of participants in clinical trials and the validity of trial results.. Web propharma has deep expertise across all signal management activities, including detection, validation, prioritization, and. Web clinical research budget plan template; Web the dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an. Risks to the safety and rights of the trial participants;. Web there are only so many. Web the content of a dsmp which clinical studies require a data and safety monitoring board (dsmb) dsmb responsibilities template. Create a standard process for testing procedures and trial management. Risks to the safety and rights of the trial participants;. Web we can prepare, or review, your safety management plan, with all associated forms including for serious adverse events (sae).. Risks to the safety and rights of the trial participants;. Web a safety management plan (smp) is a critical component of a comprehensive pharmacovigilance program. Clinical research tracking log templates. Web developing a safety management plan (smp) for pharmacovigilance the goal on the dsmp is to provide a general description of a. Web keep clinical trials on track. Web keep clinical trials on track. Web propharma has deep expertise across all signal management activities, including detection, validation, prioritization, and. Web clinical research budget plan template; Web manual of operations data & safety monitoring (dsmb, medical monitor, independent safety monitor) clinical site. Web the safety management plan (smp) is one of the most important documents in a clinical trial. Web the purpose of the dsm plan is to ensure the safety of participants in clinical trials and the validity of trial results. Risks to the safety and rights of the trial participants;. Clinical research tracking log templates;. Web find guidelines for creating data plus safety monitoring plans that include setting up procedures, producing reports, and more. Web manual of. Clinical research tracking log templates;. Safety assessment and reporting sop. Web we can prepare, or review, your safety management plan, with all associated forms including for serious adverse events (sae). Web developing a safety management plan (smp) for pharmacovigilance the goal on the dsmp is to provide a general description of a. Web clinical research budget plan template; Web according to gvp module v, the aim of a risk management plan (rmp) is to document the risk management system considered necessary to. Web manual of operations data & safety monitoring (dsmb, medical monitor, independent safety monitor) clinical site. Web the dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an. Web the safety management plan (smp) is one of the most important documents in a clinical trial. Web dsmp template updated 06may20195 data and safety monitoring plan template and guidelines (delete this) preface. Web the purpose of the dsm plan is to ensure the safety of participants in clinical trials and the validity of trial results. Web the national institute of mental health (nimh) has developed the following guidance for investigators. Web propharma has deep expertise across all signal management activities, including detection, validation, prioritization, and. Web a safety management plan (smp) is a critical component of a comprehensive pharmacovigilance program. Web management of safety information from clinical trials report of cioms working group vi geneva 2005 cioms management of safety information from. Web there are only so many clinical trial safety management plan template that can help with if there are only 3 people obtainable. Create a standard process for testing procedures and trial management. Web the content of a dsmp which clinical studies require a data and safety monitoring board (dsmb) dsmb responsibilities template. Web in clinical trials, major risks can be very broadly categorised into: Clinical research tracking log templates.Browse Our Image of Clinical Trial Safety Management Plan Template
Free Clinical Trial Templates Smartsheet
Clinical Trial Safety Management Plan Template Best Template Ideas
Clinical Trial Safety Management Plan Template Best Template Ideas
Clinical Trial Safety Management Plan Template Best Template Ideas in
Iso14971 Risk Management Template / 13 Straightforward Steps To
The enchanting The Basics Of Clinical Trial Centralized Monitoring For
Clinical Trial Safety Management Plan Template Best Template Ideas
Clinical Trial Safety Management Plan Template Best Template Ideas
Clinical Trial Safety Management Plan Template Best Template Ideas
Related Post: