Data Management Plan Template Clinical Trial
Data Management Plan Template Clinical Trial - Web dmptool is a fillable template that walks researchers through completing a plan that complies with funder. Web society for clinical data management (scdm) Clinical data is either collected during patient care or as part of a clinical trial program. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Web this data management plan template provides the required contents of a standard clinical trial data management. Rare disease patient, caregiver and advocate presentations from the fda’s “clinical. Doppler (university of city, clinical imaging data collection), mr. Web the purpose of this sop is to provide the minimum standards required to ensure all clinical trial data, from the point of. Head of data management, norwich clinical trials unit, uea approved by: Web introduction to clinical data. Web monthly with members of the project team, dr. Clinical research tracking log templates. Web the sample data management and sharing plan below is for a proposal conducting clinical research with human. Clinical data is either collected during patient care or as part of a clinical trial program. Web investigators should consider using this template when developing the data and. Head of data management, norwich clinical trials unit, uea approved by: Web introduction to clinical data. Clinical data is either collected during patient care or as part of a clinical trial program. Doppler (university of city, clinical imaging data collection), mr. Web a data management plan, or dmp, is a formal document that outlines how data will be handled during. Web dmptool is a fillable template that walks researchers through completing a plan that complies with funder. Web data management plan template. 6é÷}ha 'wïö “ö®b å˜ à¤wúí+öëîfô™.sâx. Web the purpose of this sop is to provide the minimum standards required to ensure all clinical trial data, from the point of. Clinical data is either collected during patient care or as. Head of data management, norwich clinical trials unit, uea approved by: Rare disease patient, caregiver and advocate presentations from the fda’s “clinical. Web society for clinical data management (scdm) Clinical research tracking log templates. Data handling study team agreement. Web sample plan a. Web this template is created based on experience within the association of clinical data managers (acdm) and covers most. Web clinical research budget plan template; Data handling study team agreement. Web the purpose of this sop is to provide the minimum standards required to ensure all clinical trial data, from the point of. Web this template is created based on experience within the association of clinical data managers (acdm) and covers most. Web society for clinical data management (scdm) Clinical and/or mri data from human research participants. Data handling study team agreement. Web august 7, 2023. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Data collection and documentation 1.1 what data will you collect, observe, generate or reuse ? 6é÷}ha 'wïö “ö®b å˜ à¤wúí+öëîfô™.sâx. Clinical and/or mri data from human research participants. Web analysis, data management and clinical assessment operate together, identifying and removing risk and. Data collection and documentation 1.1 what data will you collect, observe, generate or reuse ? Data handling study team agreement. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Web a data management plan, or dmp, is a formal document that outlines how data will be handled during and after a. Data collection and documentation 1.1 what data will you collect, observe, generate or reuse ? Web clinical research budget plan template; Clinical data is either collected during patient care or as part of a clinical trial program. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Clinical and/or mri data from. Web clinical research budget plan template; Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Web 11 rows templates for data management plans are based on the specific requirements listed in funder policy. Web data management plan template. Web this template is created based on experience within the association of clinical. Head of data management, norwich clinical trials unit, uea approved by: Web data management plan template. Web this template is created based on experience within the association of clinical data managers (acdm) and covers most. Web the purpose of this sop is to provide the minimum standards required to ensure all clinical trial data, from the point of. Data collection and documentation 1.1 what data will you collect, observe, generate or reuse ? 6é÷}ha 'wïö “ö®b å˜ à¤wúí+öëîfô™.sâx. Web clinical research budget plan template; Doppler (university of city, clinical imaging data collection), mr. Rare disease patient, caregiver and advocate presentations from the fda’s “clinical. Web a data management plan, or dmp, is a formal document that outlines how data will be handled during and after a research. Web sample plan a. Web introduction to clinical data. Clinical research tracking log templates. Web the sample data management and sharing plan below is for a proposal conducting clinical research with human. Data handling study team agreement. Web monthly with members of the project team, dr. Web in an effort to minimise such risks and to establish a consistent approach, this document has been developed to cover. Web this data management plan template provides the required contents of a standard clinical trial data management. Web analysis, data management and clinical assessment operate together, identifying and removing risk and enabling quality from a. Web august 7, 2023.Free Clinical Trial Templates Smartsheet for Monitoring Report
The enchanting The Basics Of Clinical Trial Centralized Monitoring For
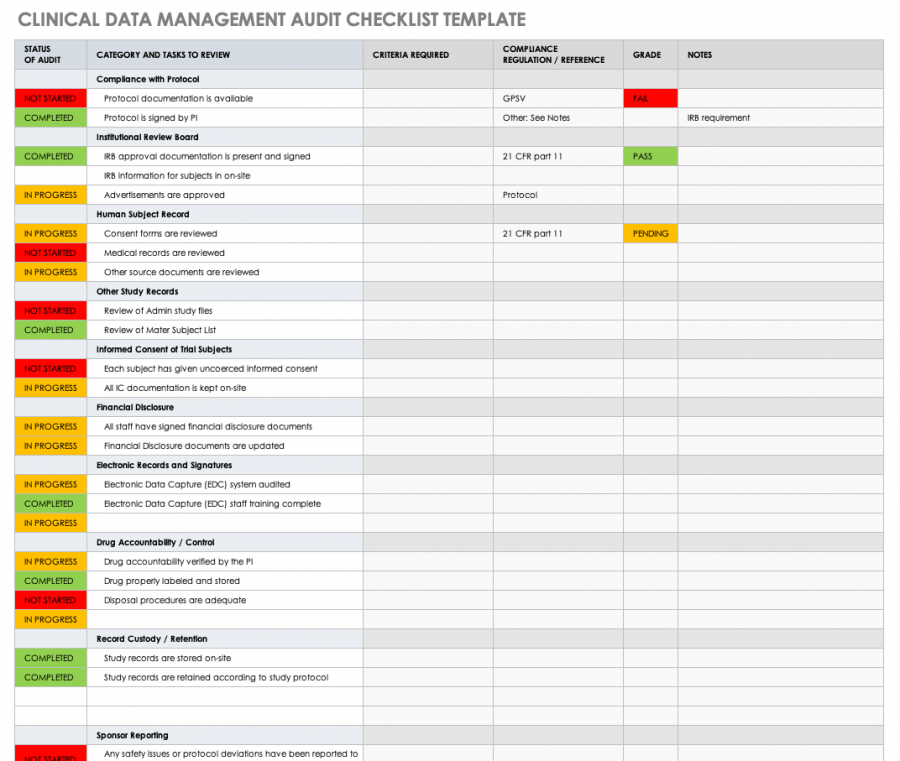
All about Clinical Trial Data Management Smartsheet
All about Clinical Trial Data Management Smartsheet
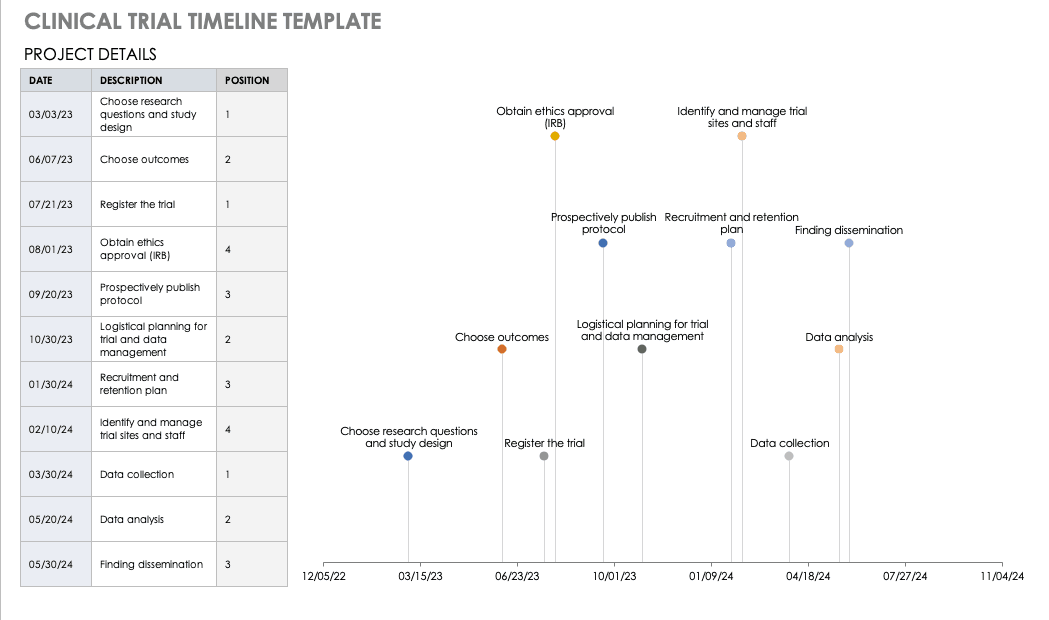
Clinical Trial Template Master of Documents
All about Clinical Trial Data Management Smartsheet
Clinical Trial Report Template New Creative Template Ideas
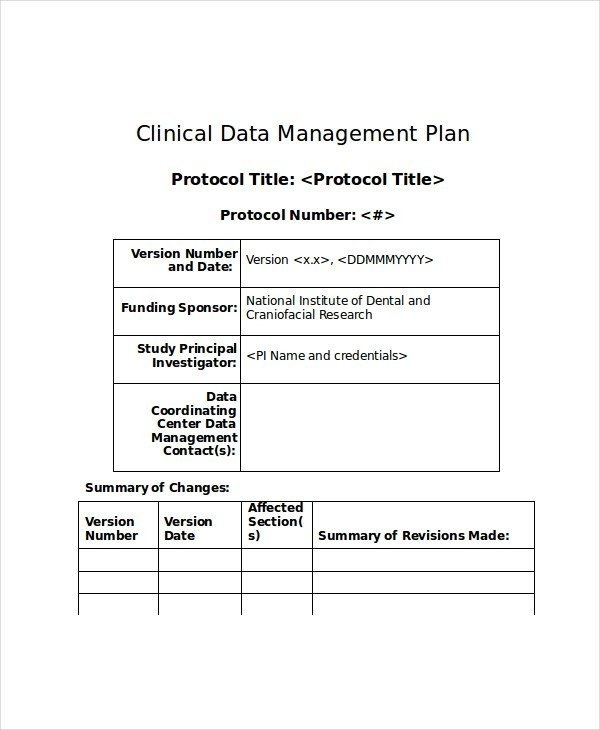
11+ Data Management Plan Examples PDF Examples
11+ Data Management Plan Examples PDF Examples
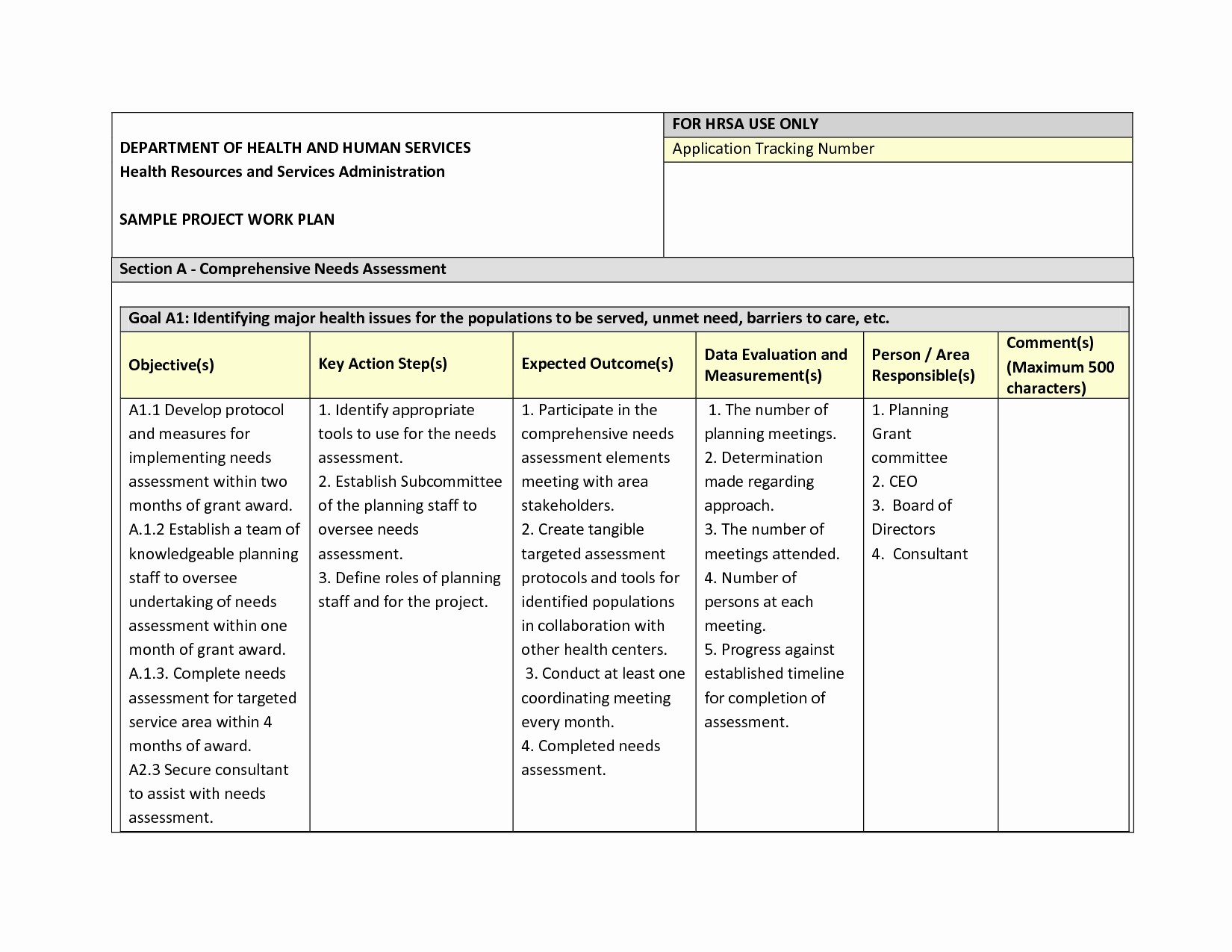
Data Management Plan Medical Research Council
Related Post: