Fda Protocol Template
Fda Protocol Template - Web this template provides the food and drug administration’s (fda) current recommendations concerning what data. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national. The electronic protocol writing tool aims to facilitate the development of. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of. Web 20 drug administration (fda) and sponsors or applicants relating to the development and review 21 of drug or biological drug. Center for drug evaluation and research, office of regulatory policy this template. Web fda updates the clinical protocol template. (thursday, january 19, 2023) the fda recently released an. Web center for drug evaluation and research mapp 5220.8 rev. Web this protocol template aims to facilitate the development of two types of clinical trials involving human. Guidance documents listed below represent the agency's. Web center for drug evaluation and research mapp 5220.8 rev. Web click the thumbnail to access a free template. Protocol concurrence will be issued solely based upon the information you provide in the qbr template. (thursday, january 19, 2023) the fda recently released an. Web 20 drug administration (fda) and sponsors or applicants relating to the development and review 21 of drug or biological drug. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of. Web this protocol template aims to facilitate the development of two types of clinical trials involving. Web this protocol template aims to facilitate the development of two types of clinical trials involving human. Office of generic drugs and office of. Web 15 rows comparison of fda, epa, oecd glp protocol & conduct; Web developed jointly by nih and the food and drug administration (fda), the protocol template provides a standard. Web fda updates the clinical protocol. The electronic protocol writing tool aims to facilitate the development of. Web 138 rows clinical trials guidance documents. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data. (thursday, january 19, 2023) the fda recently released an. The draft guidance has been. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national. Web to set this template's initial visibility, the |state= parameter may be used: Web this template. (thursday, january 19, 2023) the fda recently released an. Web center for drug evaluation and research mapp 5220.8 rev. The electronic protocol writing tool aims to facilitate the development of. Web this page includes seven different protocol templates for developing a variety of different new research protocols. Web this clinical trial protocol template is a suggested format for phase 2. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national. Web 20 drug administration (fda) and sponsors or applicants relating to the development and review 21 of drug or biological drug. Web 138 rows clinical trials guidance documents. Protocol concurrence will be issued solely based upon the information you. Center for drug evaluation and research, office of regulatory policy this template. Web this protocol template aims to facilitate the development of two types of clinical trials involving human. Web fda updates the clinical protocol template. Web click the thumbnail to access a free template. Web this clinical trial protocol template is a suggested format for phase 2 and 3. Web the fda staff responsible for this guidance as listed on the title page. Web developed jointly by nih and the food and drug administration (fda), the protocol template provides a standard. Web 15 rows comparison of fda, epa, oecd glp protocol & conduct; Web this template provides the food and drug administration’s (fda) current recommendations concerning what data. Web. The electronic protocol writing tool aims to facilitate the development of. Web 138 rows clinical trials guidance documents. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of. Web clinical trial protocols should include a clear description of trial design and patient selection criteria as well as. Office of generic drugs and office of. Web to set this template's initial visibility, the |state= parameter may be used: Guidance documents listed below represent the agency's. Web clinical trial protocols should include a clear description of trial design and patient selection criteria as well as description of. Web 15 rows comparison of fda, epa, oecd glp protocol & conduct; Web center for drug evaluation and research mapp 5220.8 rev. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data. Web this page includes seven different protocol templates for developing a variety of different new research protocols. Web this protocol template aims to facilitate the development of two types of clinical trials involving human. The draft guidance has been. The electronic protocol writing tool aims to facilitate the development of. The national institutes of health (nih) and food and drug administration (fda) developed a clinical. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of. Web 20 drug administration (fda) and sponsors or applicants relating to the development and review 21 of drug or biological drug. Web developed jointly by nih and the food and drug administration (fda), the protocol template provides a standard. Format and content of a rems document: Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national. Web fda updates the clinical protocol template. Web 138 rows clinical trials guidance documents. Center for drug evaluation and research, office of regulatory policy this template.Clinical Trial Protocol
Three steps to plan for the FDA’s new food label rules 20161018
PROCESS VALIDATION SOP Template MD46 GMP, QSR & ISO Compliance
Protocol Template 05Feb2016 508 Clinical Trial Food And Drug
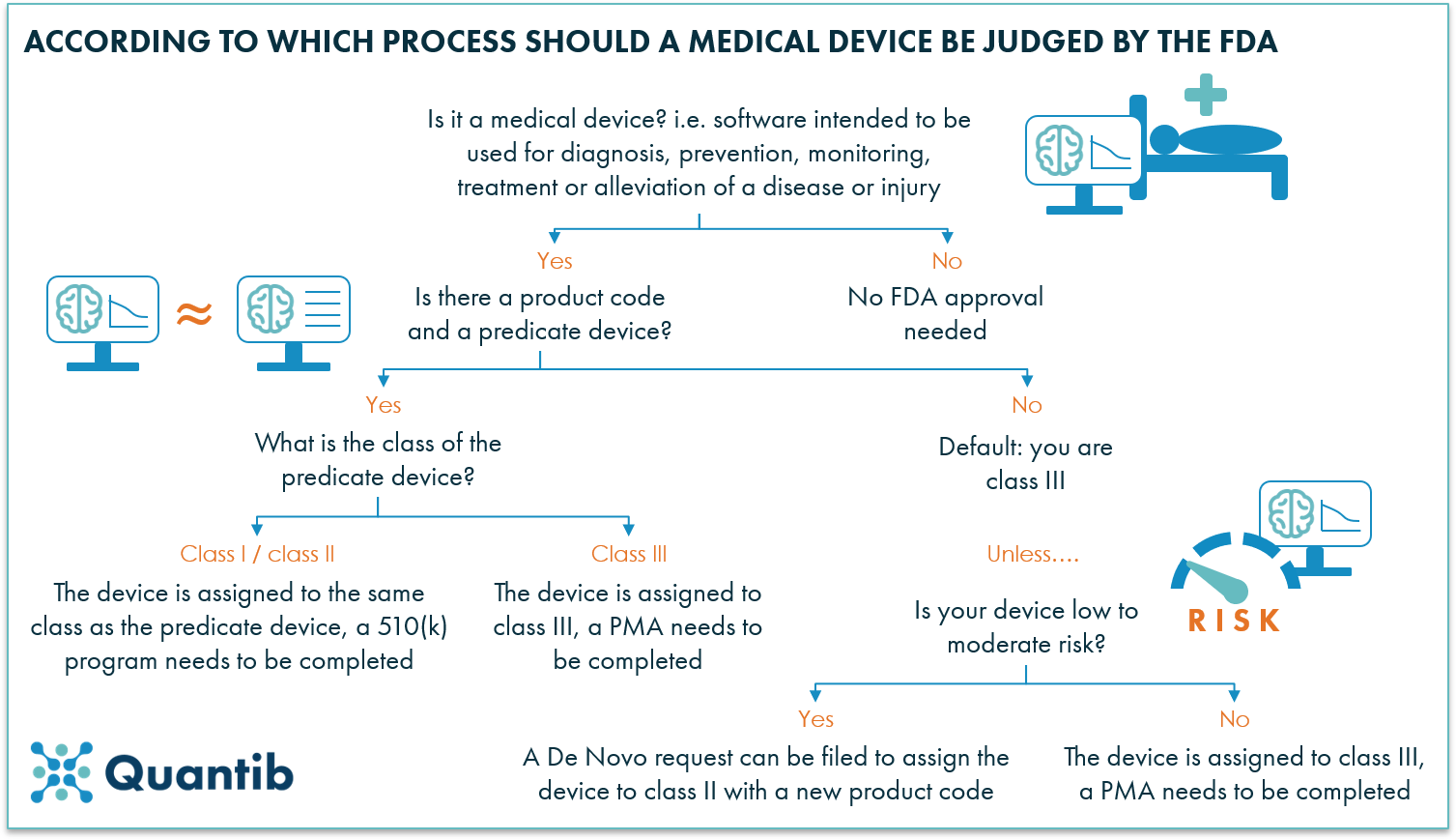
A 101 guide to the FDA regulatory process for AI radiology software
Validation Master Plan FDA EU WHO Pharma Meddevice Bio
Retail policy and procedure manual template
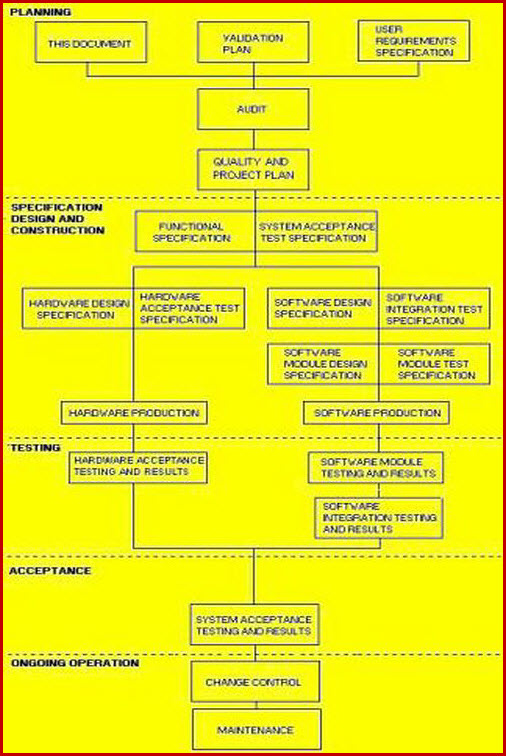
FDA Software Validation 2022 Guide, Checklist & Template
Stability Study Protocol Template williamsonga.us
Iq Oq Pq Software Validation Templates
Related Post: