Medical Device Quality Plan Template
Medical Device Quality Plan Template - However it is an essential tool that can help medical device manufacturer to deliver on specific projects. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Web quality plans are documented plans that outline quality policies, procedures, practices, and guidelines for. Web a medical device quality management system (qms) is a structured system that documents the procedures. Web in the intricate landscape of medical device development, ensuring patient safety is paramount. Web crafting a quality planner for medical device companies is difficult, and it must contain these 13 essentials if you. Web iso 13485 document template: In general, quality plan is not a specific requirements from the iso 13485:2016 or from fda quality system regulation 21 cfr 820; When building a quality assurance plan, adhering to regulatory requirements should be your. Quality plan the quality plan is the documented list of arrangements needed for the creation of. Web iso 13485 document template: Web design and development plan template (medical device per iso 13485 and 21 cfr 820) free 0 € (ex. Web med dev qms templates are proven procedures that are efficient and easy to understand. Web iso 13485 templates. Make a monthly sale of about $450,000 and about $950,000 for the first. Web design and development plan template (medical device per iso 13485 and 21 cfr 820) free 0 € (ex. Make a monthly sale of about $450,000 and about $950,000 for the first. However it is an essential tool that can help medical device manufacturer to deliver on specific projects. The document should be tailored to the specific. Web sell a. Web crafting a quality planner for medical device companies is difficult, and it must contain these 13 essentials if you. Web iso 13485 templates. The iso 13485 is the standard for quality management in the medical. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. In general,. Web this document is intended to form the basis for a supplier agreement for a medical device manufacturer. Web here we describe the 10 essential contents of a quality plan, according to iso 10005:2018. Web in the intricate landscape of medical device development, ensuring patient safety is paramount. Web this article explains how to write a quality system plan template. Web design and development plan template (medical device per iso 13485 and 21 cfr 820) free 0 € (ex. When building a quality assurance plan, adhering to regulatory requirements should be your. Each manufacturer shall establish a quality plan which defines the quality practices,. Quality plan the quality plan is the documented list of arrangements needed for the creation of.. Quality plan the quality plan is the documented list of arrangements needed for the creation of. Web here we describe the 10 essential contents of a quality plan, according to iso 10005:2018. Web a medical device project plan template can help streamline all the important steps involved in creating, designing, and. Each manufacturer shall establish a quality plan which defines. In general, quality plan is not a specific requirements from the iso 13485:2016 or from fda quality system regulation 21 cfr 820; Web this article outlines an eu mdr quality plan for compliance with european regulation 2017/745 for medical. 24, 2021 • iso 13485, regulation (eu) 2017/745 the european regulation for medical devices requires. Web a medical device quality management. In general, quality plan is not a specific requirements from the iso 13485:2016 or from fda quality system regulation 21 cfr 820; Web crafting a quality planner for medical device companies is difficult, and it must contain these 13 essentials if you. Web quality plans are documented plans that outline quality policies, procedures, practices, and guidelines for. Web med dev. The iso 13485 is the standard for quality management in the medical. Make a monthly sale of about $450,000 and about $950,000 for the first. Web iso 13485 templates. Medical device description and specification. Web this article outlines an eu mdr quality plan for compliance with european regulation 2017/745 for medical. Web sell a minimum of 5000 medical devices per day globally. Web design and development plan template (medical device per iso 13485 and 21 cfr 820) free 0 € (ex. In general, quality plan is not a specific requirements from the iso 13485:2016 or from fda quality system regulation 21 cfr 820; Web this article outlines an eu mdr quality. Web this medical devices development plan describes in detail all essential steps to be considered prior to start the development. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Quality plan the quality plan is the documented list of arrangements needed for the creation of. Web design and development plan template (medical device per iso 13485 and 21 cfr 820) free 0 € (ex. In general, quality plan is not a specific requirements from the iso 13485:2016 or from fda quality system regulation 21 cfr 820; The iso 13485 is the standard for quality management in the medical. Web here we describe the 10 essential contents of a quality plan, according to iso 10005:2018. Each manufacturer shall establish a quality plan which defines the quality practices,. Web crafting a quality planner for medical device companies is difficult, and it must contain these 13 essentials if you. Web sell a minimum of 5000 medical devices per day globally. Web this article explains how to write a quality system plan template to revise and update your quality system for compliance with iso. Web a medical device project plan template can help streamline all the important steps involved in creating, designing, and. Make a monthly sale of about $450,000 and about $950,000 for the first. Web this document is intended to form the basis for a supplier agreement for a medical device manufacturer. Web a medical device quality management system (qms) is a structured system that documents the procedures. However it is an essential tool that can help medical device manufacturer to deliver on specific projects. The document should be tailored to the specific. Medical device description and specification. Web in the intricate landscape of medical device development, ensuring patient safety is paramount. When building a quality assurance plan, adhering to regulatory requirements should be your.Medical Device Quality Agreement US
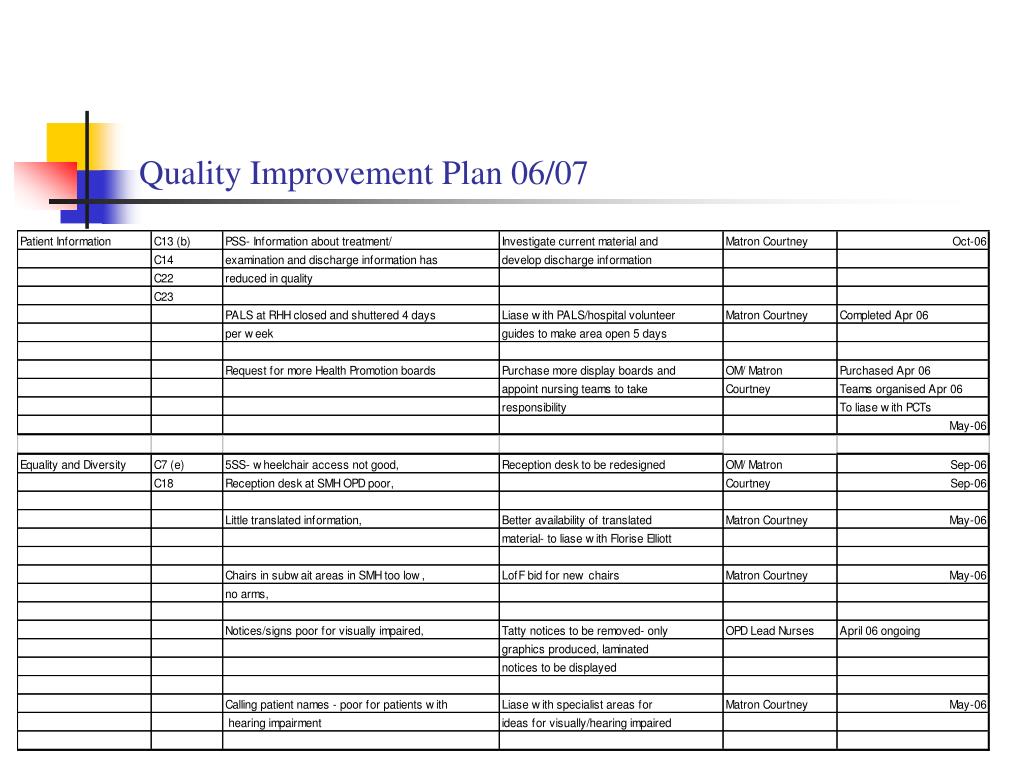
PPT Listening to Patients. OUTPATIENT QUALITY IMPROVEMENT PLAN 06/07
Installation Qualification IQ Procedure
Supplier Quality Manual Template Get Free Templates
Calibration System Procedure
Addictionary
Corrective and Preventive Action Procedure
Medical Device Clinical Investigation Plan (CIP) ISO 141552020 Compliant
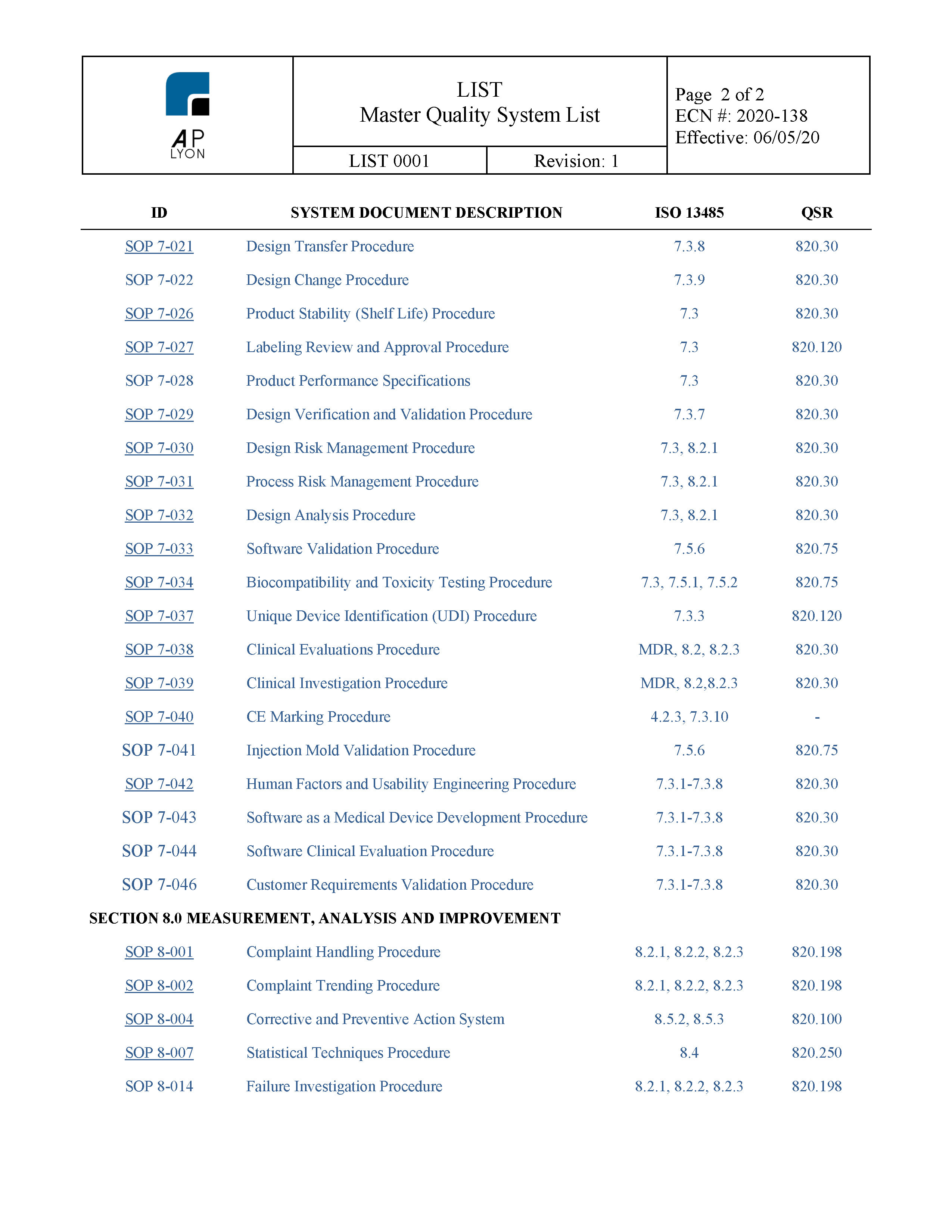
Md 001medicaldevicesdesigncontrolsop1.022
Medical Device Quality Management System DESIGN PLUS
Related Post: