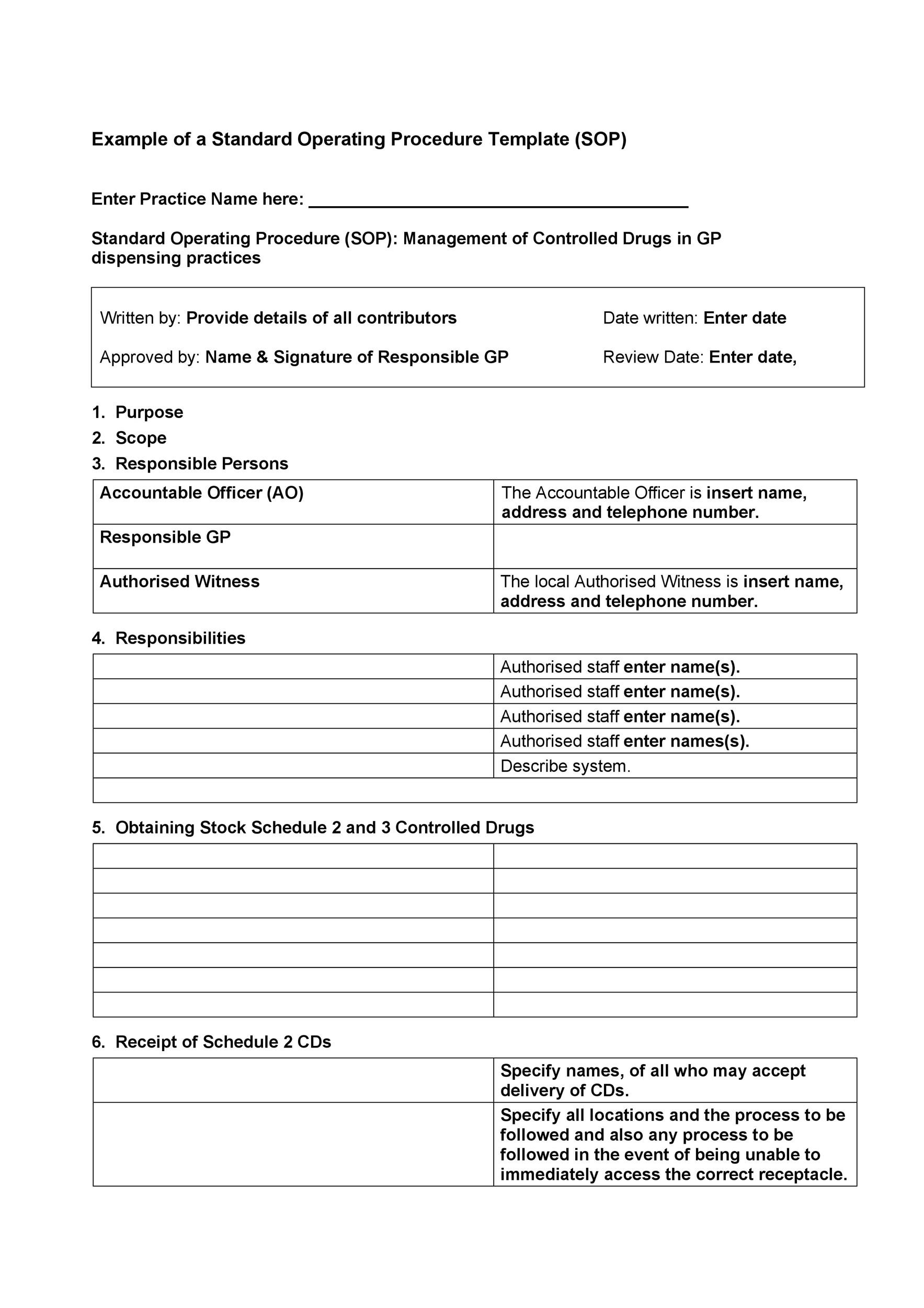
Medical Device Sop Templates
Medical Device Sop Templates - These mdsap regulatory authority quality management system (qms). Introduction short description of the product (name, composition, classification, intended. Group md200 design and document controls sop; Web when medical device academy writes standard operating procedures, we use a standard template for the sections. Web medical device qms document templates. All document templates for medical device. Web medical device design control sop 199,00 € add to cart iso 13485 and 21 cfr 820, cfr 4 standard operating procedure templates. Web design history file sop; Md21 device master record sop; Medical device human factor sop; Our extensive expertise in developing sop templates will make your life a breeze. Their templates are not free, and the prices vary. Some of the templates they offer include: It will help you achieve conformity with most requirements in iso 14971:2019. Web sop templates for medical device and pharmaceutical manufacturers gmprocedures are time saving sop. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Web standard operating procedure (sop) for risk management according to en iso 14971:2019. Web this sop describes the development of medical devices in accordance with regulatory requirements. Group md200 design and document controls sop; Comprehensive iso 13485 sop. Document and change controls sop; Medical device human factor sop; Web iso 13485 template for medical devices and training package. Their templates are not free, and the prices vary. Web i will provide you with all the package you need to write procedures for a medical device company (free procedure. Group md200 design and document controls sop; Comprehensive iso 13485 sop overview and implementation. These mdsap regulatory authority quality management system (qms). Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Web the fda qsr establishes the requirements for medical device quality systems, including requirements for medical. Md21 device master record sop; Web medical device design control sop 199,00 € add to cart iso 13485 and 21 cfr 820, cfr 4 standard operating procedure templates. Web iso 13485 templates. Web design history file sop; Some of the templates they offer include: The iso 13485 is the standard for quality management in the medical. Web when medical device academy writes standard operating procedures, we use a standard template for the sections. Web this sop describes the development of medical devices in accordance with regulatory requirements. These mdsap regulatory authority quality management system (qms). Document and change controls sop; Web medical device qms document templates. Web gmp labeling offers several templates for sop medical device design and document controls. All document templates for medical device. Web this sop describes the development of medical devices in accordance with regulatory requirements. Document and change controls sop; Comprehensive iso 13485 sop overview and implementation. Web iso 13485 templates. Web standard operating procedure (sop) for risk management according to en iso 14971:2019. We have a selection of free and premium templates available ranging from checklists to hazard traceability matrixes. Md21 device master record sop; Web medical device design control sop 199,00 € add to cart iso 13485 and 21 cfr 820, cfr 4 standard operating procedure templates. Web mdsap qms procedures and forms. Web this sop describes the development of medical devices in accordance with regulatory requirements. Web iso 13485 template for medical devices and training package. Web a library of free medical device. Web mdsap qms procedures and forms. Document and change controls sop; Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Medical device human factor sop; Web iso 13485 templates. Md21 device master record sop; Web and here’s the cherry on top: Web sop templates for medical device and pharmaceutical manufacturers gmprocedures are time saving sop. Their templates are not free, and the prices vary. Web the fda qsr establishes the requirements for medical device quality systems, including requirements for medical device. Web iso 13485 templates. Web i will provide you with all the package you need to write procedures for a medical device company (free procedure. Web iso 13485 template for medical devices and training package. All document templates for medical device. Web standard operating procedure (sop) for risk management according to en iso 14971:2019. Document and change controls sop; Introduction short description of the product (name, composition, classification, intended. Our extensive expertise in developing sop templates will make your life a breeze. We have a selection of free and premium templates available ranging from checklists to hazard traceability matrixes. Web mdsap qms procedures and forms. These mdsap regulatory authority quality management system (qms). The iso 13485 is the standard for quality management in the medical. Web when medical device academy writes standard operating procedures, we use a standard template for the sections. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Web this sop describes the development of medical devices in accordance with regulatory requirements.FREE 61+ SOP Templates in PDF MS Word
LABELING CONTROLS SOP Template MD55 GMP, QSR & ISO Compliance
37 Best Standard Operating Procedure (SOP) Templates
SOP on Standard Operating Procedures Standard operating procedure
RECEIVING & INSPECTION SOP Template MD59 GMP, QSR & ISO Comp
FREE 35+ SOP Templates in PDF
Download SOP Templates 16 in 2021 Sop template, Standard operating
MEDICAL DEVICE REPORTING SOP Template MD33 GMP, QSR & ISO Comp
SERVICING SOP Template MD31 GMP, QSR & ISO Compliance
11 Editable Standard Operating Procedure Template SampleTemplatess
Related Post: