Note To File Template Clinical Research
Note To File Template Clinical Research - Web study documentation tools as per the international conference on harmonization good clinical practice (ich gcp) guideline. Identify a discrepancy or problem in the conduct of the clinical research study; They may be useful, but not. Web note to file examples: Web over the years, the use of notes to file has evolved from a last resort solution to a common working practice amongst. Web welcome to global health trials' tools and templates library. Web a note to file is written to: Please note that this page has been updated for 2015 following a. Web valid notes to file (ntfs) should, at minimum, meet the following basic criteria: [insert date of the ; Identify a discrepancy or problem in the conduct of the clinical research study; Web a note to file (ntf) is a record that allows the clinical site and the sponsor to document an identified issue or. Web a note to the study file should be retained and stored, as follows: Web over the years, the use of notes to file. Web note to file examples: Web welcome to global health trials' tools and templates library. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by nih that are being. Web a note to the study file should be retained and stored, as follows: Web a note to file (ntf) is a. Please note that this page has been updated for 2015 following a. Identify a discrepancy or problem in the conduct of the clinical research study; Kept on file in the site regulatory file and made available to the. [insert date of the ; Web a note to file is written to: Web note to file examples: Please note that this page has been updated for 2015 following a. Web valid notes to file (ntfs) should, at minimum, meet the following basic criteria: No more notes to file’ by carl anderson, applied clinical trials, march 2008 “a common document at many clinical sites is the memo. Web a note to file is. Web over the years, the use of notes to file has evolved from a last resort solution to a common working practice amongst. Example 1 (doc) example 2 (doc) example 3 (doc) Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by nih that are being. Web a note to the. [insert date of the ; Web a note to file (ntf) is a record that allows the clinical site and the sponsor to document an identified issue or. Web this toolkit of materials contains templates, sample forms and information materials to assist clinical investigators in the. Identify a discrepancy or problem in the conduct of the clinical research study; Example. Web notes to the study file should be written by the individual responsible for its content, and the author should sign and date the note. Please note that this page has been updated for 2015 following a. Ote to file (ntf)] clinical research site. Web this toolkit of materials contains templates, sample forms and information materials to assist clinical investigators. Web a note to the study file should be retained and stored, as follows: Kept on file in the site regulatory file and made available to the. Web this toolkit of materials contains templates, sample forms and information materials to assist clinical investigators in the. Web for clinical researchers, the note to file, or ntf, is the mosquito that you. Ote to file (ntf)] clinical research site. Web in addition, if a data management center (dmc) is handling the data management of the clinical research. Web notes to the study file should be written by the individual responsible for its content, and the author should sign and date the note. Example 1 (doc) example 2 (doc) example 3 (doc) Web. Web for clinical researchers, the note to file, or ntf, is the mosquito that you can hear under the covers or the flat tire on the way to the. Web study documentation tools as per the international conference on harmonization good clinical practice (ich gcp) guideline. Web “we also note that, according to your january 10, 2011, response to the. Identify a discrepancy or problem in the conduct of the clinical research study; Web in addition, if a data management center (dmc) is handling the data management of the clinical research. Ote to file (ntf)] clinical research site. Web note to file examples: [insert date of the ; Please note that this page has been updated for 2015 following a. Web this toolkit of materials contains templates, sample forms and information materials to assist clinical investigators in the. Web study documentation tools as per the international conference on harmonization good clinical practice (ich gcp) guideline. Web “we also note that, according to your january 10, 2011, response to the form fda 483 and your april 27, 2010,. Web valid notes to file (ntfs) should, at minimum, meet the following basic criteria: No more notes to file’ by carl anderson, applied clinical trials, march 2008 “a common document at many clinical sites is the memo. Web notes to the study file should be written by the individual responsible for its content, and the author should sign and date the note. Web from ‘note to self: Web for clinical researchers, the note to file, or ntf, is the mosquito that you can hear under the covers or the flat tire on the way to the. Web a note to the study file should be retained and stored, as follows: Kept on file in the site regulatory file and made available to the. Example 1 (doc) example 2 (doc) example 3 (doc) Web a note to file (ntf) is a record that allows the clinical site and the sponsor to document an identified issue or. Web over the years, the use of notes to file has evolved from a last resort solution to a common working practice amongst. Web welcome to global health trials' tools and templates library.Note To File Template Download by Pharma Student Issuu
Sample Research Protocol PDF Clinical Trial Risk
PPT Orientation for New Clinical Research PERSONNEL Module 2
10+ Progress Note Templates PDF, DOC Free & Premium Templates
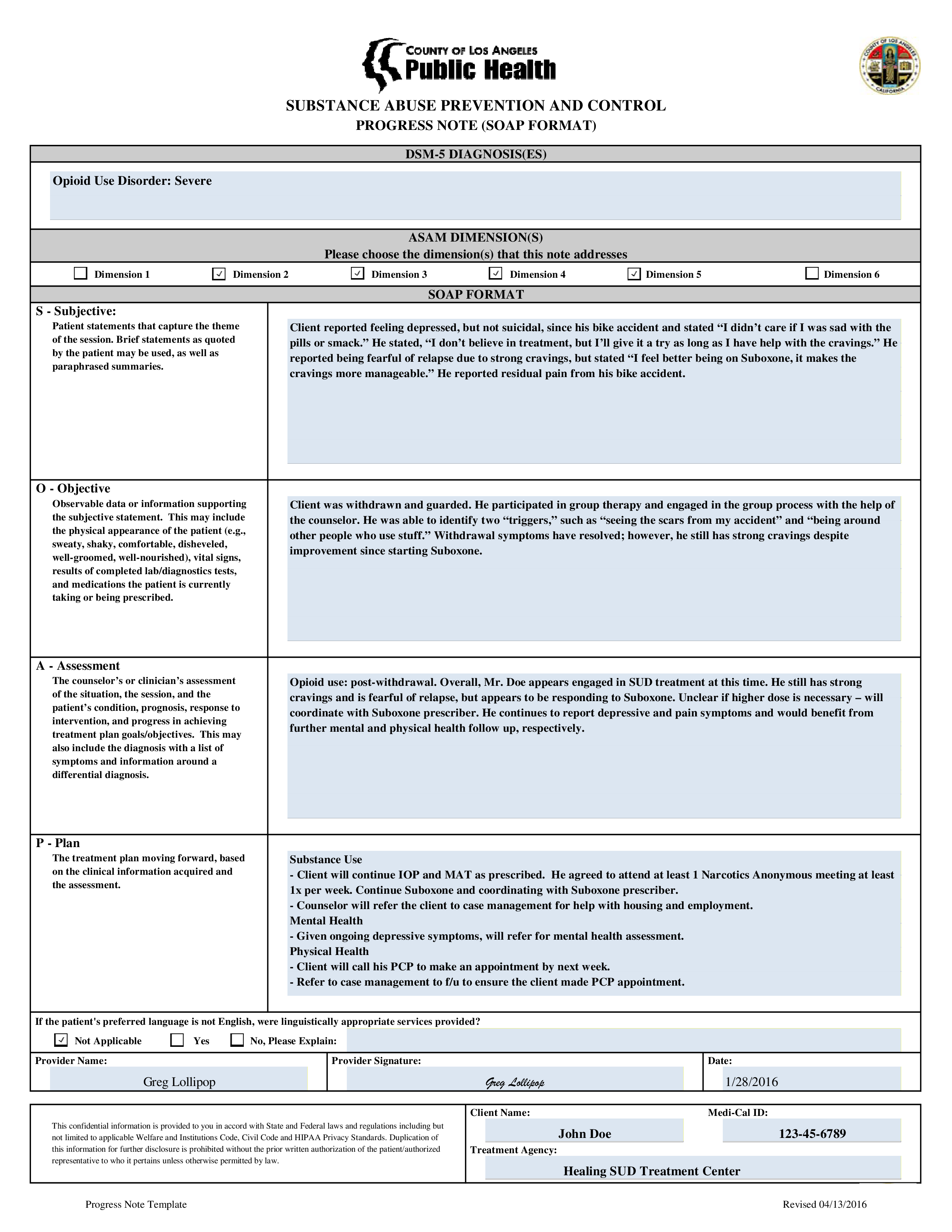
Template For Clinical Soap Note Format printable pdf download
Clinical Trial Report Template (1) TEMPLATES EXAMPLE TEMPLATES
NoteToFile Template
Clinical Note Template Collection
Clinical Progress Note Templates at
Clinical Workflow Analysis Template Template 2 Resume Examples
Related Post: