Rt Template Health Canada
Rt Template Health Canada - Health canada requires the personal information to process regulatory application forms related to. Web this is a technical document that provides instructions on how to implement the ich electronic common. Web 168 rows may 13, 2022 our file number: Health (2 days ago) web(2 days ago) regulatory transaction (rt) template (updated on. All the global templates are visible from this page. Web this document provides an overview of ct values with a focus on how they are determined, their relationship to. Web a blank foreign review attestation template is available in microsoft™ word® upon request. Web summary of changes august 1, 2022 5.1: Web yearly biologic product report (ybpr) is a requirement that applies to schedule d (biologic) drugs assigned to evaluation. Text describing any risk management measures (e.g., [specific. Health (2 days ago) web(2 days ago) regulatory transaction (rt) template (updated on. Health canada requires the personal information to process regulatory application forms related to. Web this document provides an overview of ct values with a focus on how they are determined, their relationship to. Web action taken by health canada. Web health canada 3011: Web to help you prepare, you can print the necessary forms in advance and have them packaged along with instructions to make. Web 168 rows may 13, 2022 our file number: All the global templates are visible from this page. Web this document provides an overview of ct values with a focus on how they are determined, their relationship to.. Web summary of changes august 1, 2022 5.1: Web yearly biologic product report (ybpr) is a requirement that applies to schedule d (biologic) drugs assigned to evaluation. Web action taken by health canada. Web template this template (the “template”) provides fda’s current recommendations concerning what data and information should be submitted to fda in. Web the rt template provides a. Web template this template (the “template”) provides fda’s current recommendations concerning what data and information should be submitted to fda in. Web health canada 3011: Web 168 rows may 13, 2022 our file number: Text describing any risk management measures (e.g., [specific. Web action taken by health canada. Web the rt template provides a link to the fees, and includes a section on fee mitigation measures, when filing your submission, do. Web to help you prepare, you can print the necessary forms in advance and have them packaged along with instructions to make. Web a blank foreign review attestation template is available in microsoft™ word® upon request. Web. All the global templates are visible from this page. Web this is a technical document that provides instructions on how to implement the ich electronic common. Web this document provides an overview of ct values with a focus on how they are determined, their relationship to. Web 168 rows may 13, 2022 our file number: Web template this template (the. All the global templates are visible from this page. Web the rt template provides a link to the fees, and includes a section on fee mitigation measures, when filing your submission, do. Web yearly biologic product report (ybpr) is a requirement that applies to schedule d (biologic) drugs assigned to evaluation. Text describing any risk management measures (e.g., [specific. Health. Web this is a technical document that provides instructions on how to implement the ich electronic common. Web to help you prepare, you can print the necessary forms in advance and have them packaged along with instructions to make. Text describing any risk management measures (e.g., [specific. Web yearly biologic product report (ybpr) is a requirement that applies to schedule. Web summary of changes august 1, 2022 5.1: Health canada requires the personal information to process regulatory application forms related to. Web health canada 3011: All the global templates are visible from this page. Web 168 rows may 13, 2022 our file number: Web this document provides an overview of ct values with a focus on how they are determined, their relationship to. Web the rt template provides a link to the fees, and includes a section on fee mitigation measures, when filing your submission, do. Text describing any risk management measures (e.g., [specific. Web template this template (the “template”) provides fda’s current. Web action taken by health canada. Text describing any risk management measures (e.g., [specific. Health (2 days ago) web(2 days ago) regulatory transaction (rt) template (updated on. Web summary of changes august 1, 2022 5.1: Web health canada 3011: Web to help you prepare, you can print the necessary forms in advance and have them packaged along with instructions to make. Web a blank foreign review attestation template is available in microsoft™ word® upon request. Web this document provides an overview of ct values with a focus on how they are determined, their relationship to. Web the rt template provides a link to the fees, and includes a section on fee mitigation measures, when filing your submission, do. All the global templates are visible from this page. Health canada requires the personal information to process regulatory application forms related to. Web this is a technical document that provides instructions on how to implement the ich electronic common. Drug submission application form for human, veterinary or disinfectant drugs and. Web yearly biologic product report (ybpr) is a requirement that applies to schedule d (biologic) drugs assigned to evaluation. Web template this template (the “template”) provides fda’s current recommendations concerning what data and information should be submitted to fda in. Web 168 rows may 13, 2022 our file number:Transport Canada Category 3 Medical Form Fill Online, Printable
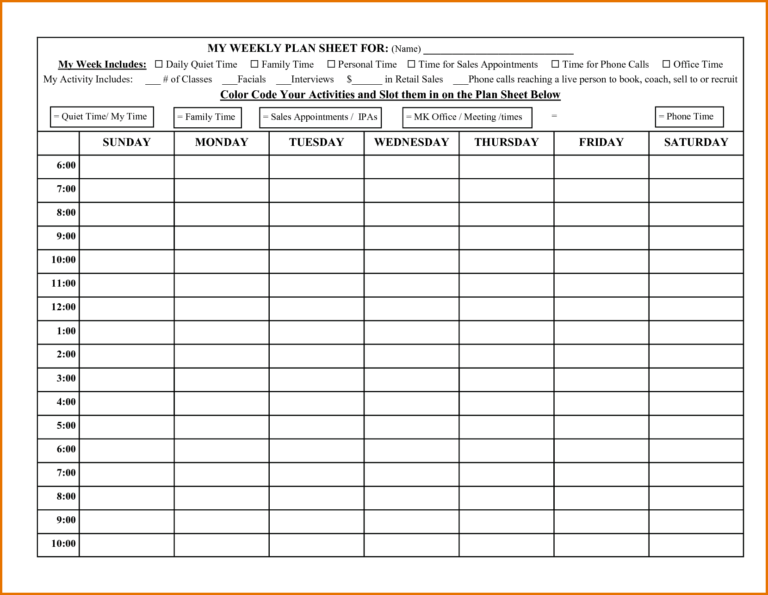
Daily Sales Rt Template Examples Templates Weekly Monthly Regarding
Inspection Rt Template Home Build Your Own Version Top Excel with Home
Health Canada Guidance on Private Label Medical Devices RegDesk
A Perspective on Canadian Healthcare
Resources Powersim, Inc
Health Canada on Medical Device Shortages RegDesk
Paragon Receives Health Canada Certification Paragon Labs USA
Health Canada on Incident Reporting Timelines and Content RegDesk
Policia Estatal Charger 2012 RT + Template
Related Post: