Validation Protocol Template
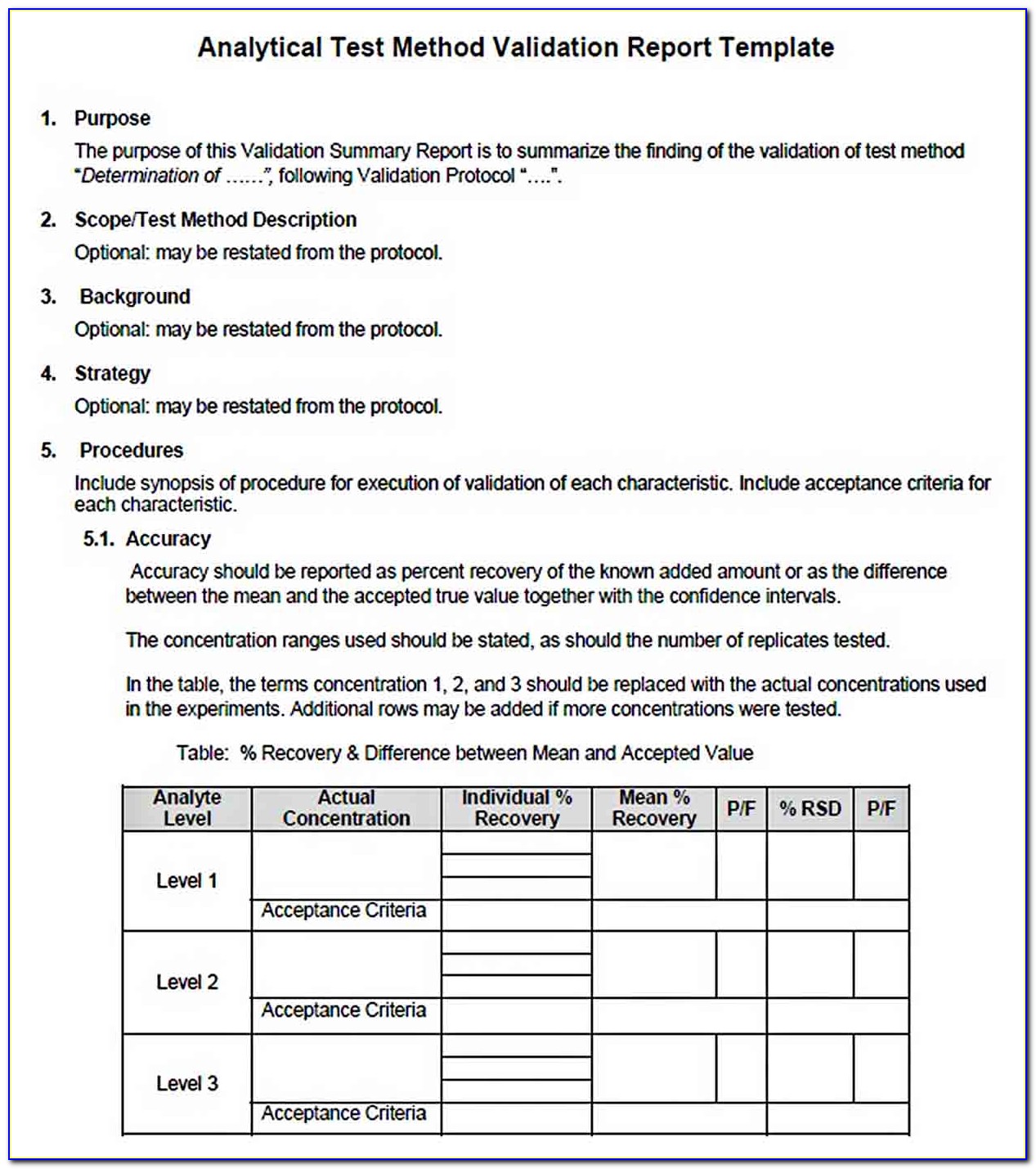
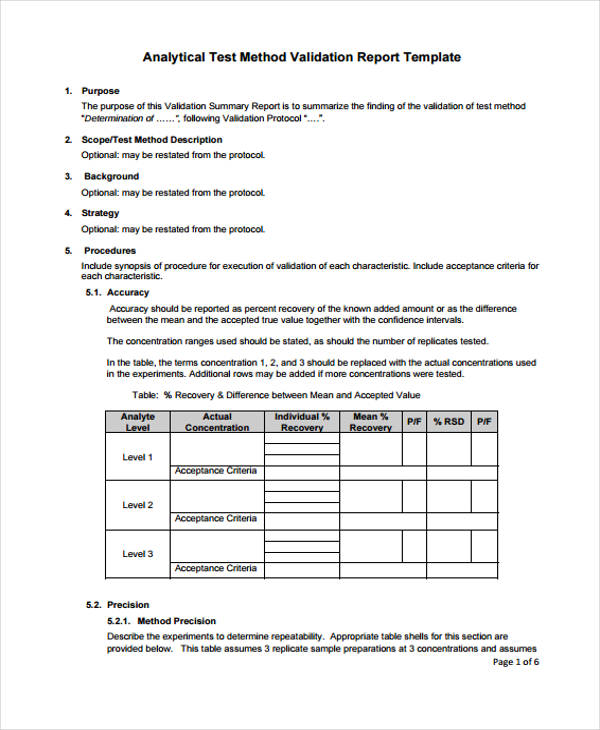
Validation Protocol Template - Templates repository for software development process (click here) or. More templates to download on the: Web sop for template for process validation protocol in pharma. These are the abbreviations we use in the medical device industry for the three steps of. Web at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. Web process validation protocol template or format for the products manufactured in the pharmaceutical product manufacturing. Cleaning validation is a procedure of establishing evidence that. However, validation plans, experiments, results and. Web template for an example methods validation protocol 171 i. Sop for process validation is a. Web what is iq, oq, pq? Web pdf template, an installation qualification template is used to complete the process validation protocol by properly. Cleaning validation is a procedure of establishing evidence that. Web sop for template for process validation protocol in pharma. Sop for process validation is a. Web process validation protocol template or format for the products manufactured in the pharmaceutical product manufacturing. Cleaning validation is a procedure of establishing evidence that. These are the abbreviations we use in the medical device industry for the three steps of. Web here are the details of validation protocol & report format + types pdf ppt. Web pdf template, an. Web use this process validation report template in the pharmaceutical industry to document everything properly. Web process validation protocol template or format for the products manufactured in the pharmaceutical product manufacturing. Define the facilities, systems, equipment, or processes in the scope of the. Web 1 objective the objective of this protocol is to define the procedure used and the acceptance. Web what is iq, oq, pq? Study this protocol was generated and approved to. More templates to download on the: Web template for process validation protocol objective to provide documented evidence with high degree of. Web develop working processes with a process validation protocol template. Study this protocol was generated and approved to. Analytical validation seeks to demonstrate that the. Web 1 objective the objective of this protocol is to define the procedure used and the acceptance criteria for validation of [insert full product description (eg. Validation studies, stability studies, and clinical studies are examples of written. Web pdf template, an installation qualification template is. Web template for process validation protocol objective to provide documented evidence with high degree of. Web process validation protocol template or format for the products manufactured in the pharmaceutical product manufacturing. Web sop for template for process validation protocol in pharma. Cleaning validation is a procedure of establishing evidence that. Web an equipment validation protocol is a written plan stating. Web use this process validation report template in the pharmaceutical industry to document everything properly. Web validation strategy this process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg). More templates to download on the: Web process validation protocol template or format for the products manufactured in the pharmaceutical product manufacturing. Web published 4 may 2023. Web validation strategy this process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg). These are the abbreviations we use in the medical device industry for the three steps of. Web template for an example methods validation protocol 171 i. Web 1 objective the objective of this protocol is to define the procedure used and the. Web validation strategy this process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg). Web this verification is achieved by subjecting the equipment to a range of fully documented inspections and tests. Use this process validation protocol template to. Validation studies, stability studies, and clinical studies are examples of written. However, validation plans, experiments, results and. These are the abbreviations we use in the medical device industry for the three steps of. Study this protocol was generated and approved to. It details factors such as product characteristics, production equipment, test scripts and methods, test parameters and acceptance criteria, test checksheets and final approval. Web template for process validation protocol objective to provide documented evidence with high. Web tance criteria for making conclusions. Web process validation protocol template or format for the products manufactured in the pharmaceutical product manufacturing. Web this verification is achieved by subjecting the equipment to a range of fully documented inspections and tests. Web pdf template, an installation qualification template is used to complete the process validation protocol by properly. Templates repository for software development process (click here) or. More templates to download on the: Web an equipment validation protocol is a written plan stating how equipment qualification will be conducted. Web at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. Use this process validation protocol template to. Web published 4 may 2023 what is cleaning validation? It details factors such as product characteristics, production equipment, test scripts and methods, test parameters and acceptance criteria, test checksheets and final approval. Web validation strategy this process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg). Web sop for template for process validation protocol in pharma. Web develop working processes with a process validation protocol template. Web use this process validation report template in the pharmaceutical industry to document everything properly. These are the abbreviations we use in the medical device industry for the three steps of. However, validation plans, experiments, results and. Web clia rules and guidance3 are silent on minimum requirements for validation protocols; Web template for an example methods validation protocol 171 i. Cleaning validation is a procedure of establishing evidence that.Analytical Method Validation Protocol Sample
Template for Process Validation Protocol Verification And Validation
Analytical Test Method Validation Protocol Template
Software Validation Templates
Tem290 Process Validation Protocol Template Sample Verification And
Excel Spreadsheet Validation Protocol Template
process validation protocol DriverLayer Search Engine
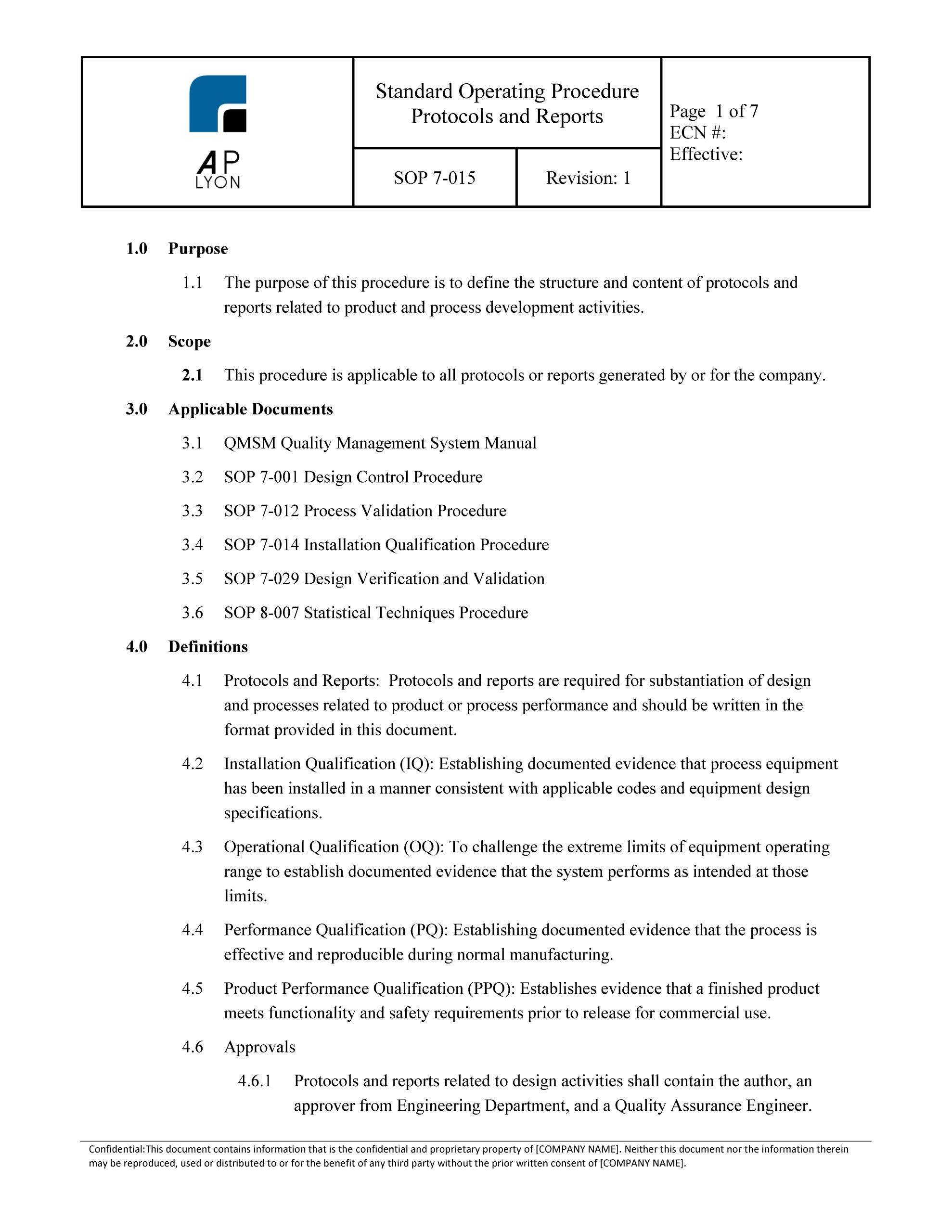
Validation Protocols Reports Procedure
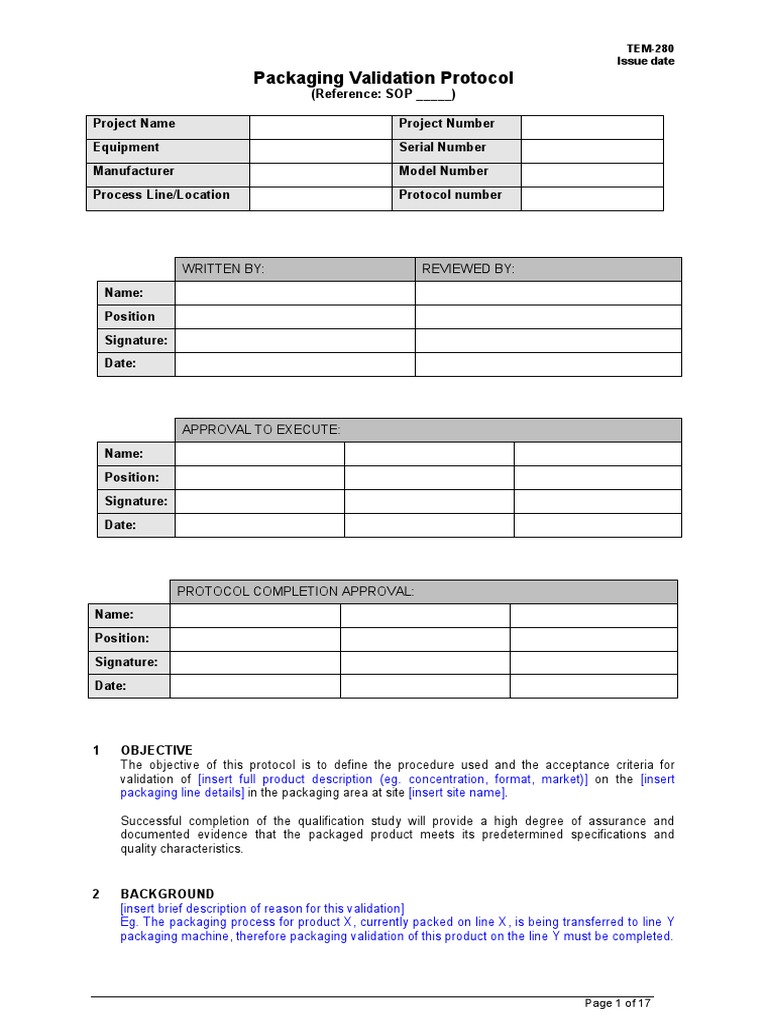
TEM280 Packaging Validation Protocol Template Sample Verification
TEM260_Cleaning_Validation_Protocol_Template_sample.pdf Chemistry
Related Post: